Proteolytic Systems Activated During Calorie Restriction
Understanding ubiquitin-proteasome and autophagy degradation pathways
Understanding ubiquitin-proteasome and autophagy degradation pathways

During energy restriction, muscle proteins are degraded to liberate amino acids for gluconeogenesis and other essential metabolic functions. This degradation occurs through two primary pathways: the ubiquitin-proteasome system (UPS) and macroautophagy. Both are upregulated in response to low energy status, creating a coordinated increase in protein catabolism.
The regulation of these pathways is mediated by transcription factors and signalling kinases that sense the energy deficit and activate gene expression programmes for degradative machinery.
The UPS is a selective degradation pathway that targets specific proteins tagged with ubiquitin chains. The process involves three enzymatic steps:
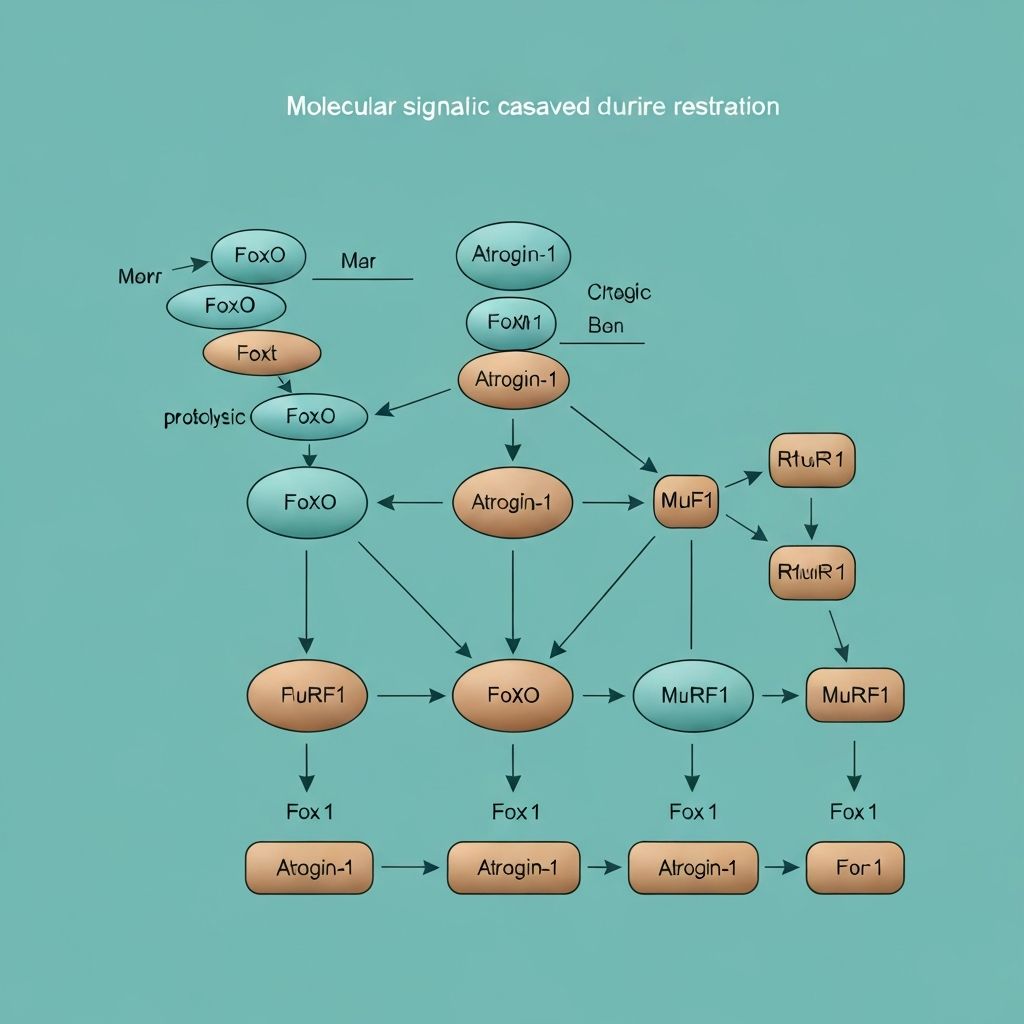
In muscle, the primary E3 ligases involved in protein degradation during energy deficit are atrogin-1 (MAFbx) and MuRF1 (muscle RING-finger protein 1). These muscle-specific ubiquitin ligases are upregulated at the transcriptional level by the FoxO (forkhead box O) transcription factors.
FoxO activation occurs when mTORC1 is inhibited and when FOXO is dephosphorylated by the phosphatase PTEN. During energy deficit, both conditions are met: mTORC1 is suppressed by low energy status, and AMPK-mediated signalling inhibits Akt, which normally phosphorylates and inactivates FoxO. As a result, FoxO translocates to the nucleus, where it binds to promoter regions of atrogin-1 and MuRF1 genes, driving their transcription.
The result is an ~5- to 15-fold increase in atrogin-1 and MuRF1 expression during energy restriction, leading to enhanced polyubiquitination and proteasomal degradation of myofibrillar proteins.
Macroautophagy (commonly referred to as autophagy) is a non-selective, bulk degradation process whereby cytoplasmic components are sequestered within double-membrane organelles called autophagosomes and delivered to lysosomes for hydrolysis. In muscle, autophagy is upregulated during energy deficit, catabolic states, and extended fasting.
The initiation of autophagy is controlled by the ULK1 (unc-51-like kinase 1) complex, which is inhibited by mTORC1 under anabolic conditions. When mTORC1 is suppressed (as in energy deficit), ULK1 becomes active and phosphorylates downstream effectors including Atg13 and FIP200, triggering the formation of the autophagosomal membrane.
AMPK, which is activated during energy deficit due to increased AMP/ATP ratio, further promotes autophagy by phosphorylating TSC2 (which inhibits mTORC1) and by directly phosphorylating ULK1 at different sites than mTORC1. This dual regulation ensures robust autophagy activation when cellular energy is limited.
Autophagic degradation in muscle leads to the breakdown of myofibrillar proteins and the release of amino acids into the cytoplasm, where they can be utilized for gluconeogenesis or incorporated into newly synthesised proteins in other tissues. The selectivity of autophagic degradation can be partially directed toward damaged or dysfunctional organelles and proteins through a process called selective autophagy, though bulk autophagy predominates in energy deficit.
AMP-activated protein kinase (AMPK) acts as a cellular energy sensor, becoming activated when the AMP/ATP ratio increases, indicating low energy charge. During energy restriction, AMPK phosphorylation increases markedly. Once activated, AMPK phosphorylates and inactivates acetyl-CoA carboxylase (ACC), which leads to reduced malonyl-CoA and increased fatty acid oxidation—a catabolic adaptation to mobilise energy.
Additionally, AMPK phosphorylates TSC2, which strongly inhibits mTORC1. This prevents anabolic processes (protein synthesis, lipid synthesis) while AMPK simultaneously promotes catabolic processes through ULK1 activation and enhanced autophagy.
AMPK also phosphorylates PGC-1α, promoting mitochondrial biogenesis and oxidative capacity—adaptations that enhance the ability to oxidise substrates for energy during restriction.
Resistance training, by activating mTORC1 and Akt signalling, inhibits FoxO and thereby suppresses the transcription of atrogin-1 and MuRF1. This represents a key mechanism by which mechanical loading protects against excessive protein degradation during energy deficit.
Furthermore, mTORC1 activation inhibits ULK1, reducing the initiation of autophagy. Thus, muscles experiencing resistance training maintain lower autophagy rates compared to untrained muscles during energy restriction.
The combined effect is that resistance exercise creates a local muscular environment in which proteolytic pathways are suppressed relative to untrained conditions, partially offsetting the systemic catabolic signals from energy deficit.